Interfacial Stability of Lithium Sulfur Batteries
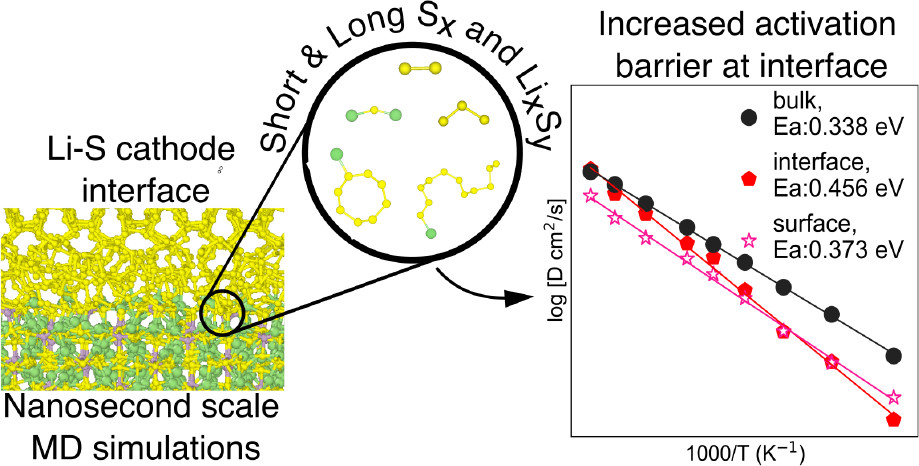
Congratulations to Manas Likhit Holekevi Chandrappa, Ji Qi, Chi Chen and Swastika Banerjee on the publication of “Thermodynamics and Kinetics of the Cathode−Electrolyte Interface in All-Solid-State Li−S Batteries” in the Journal of the American Chemical Society! Lithium−sulfur batteries (LSBs) use cheap and abundant sulfur in place of expensive metal-based cathodes. Using a solid electrolyte in place of traditional liquid electrolytes mitigates polysulfide shuttling, a key impediment to LSB commercialization. In this work, we present a comprehensive study of the thermodynamics and kinetics of the cathode−electrolyte interface in all-solid-state LSBs. Using DFT calculations, we show that among the major solid electrolyte chemistries (oxides, sulfides, nitrides, and halides), sulfides are the most stable solid electrolytes against the S cathode, as well as the most promising buffer layers if the use of other SE chemistries is desired. Finally, MD simulations with an accurate machine learning interatomic potential revealed that the most stable Li3PS4(100)/S interfaces form 2D channels with lower activation barriers for Li diffusion. These results provide critical new insights into the cathode−electrolyte interface design for next-generation all-solid-state LSBs. We gratefully acknowledge Nissan Motor Inc and Nissan North America for their generous support for this work! This work has been published open access […]