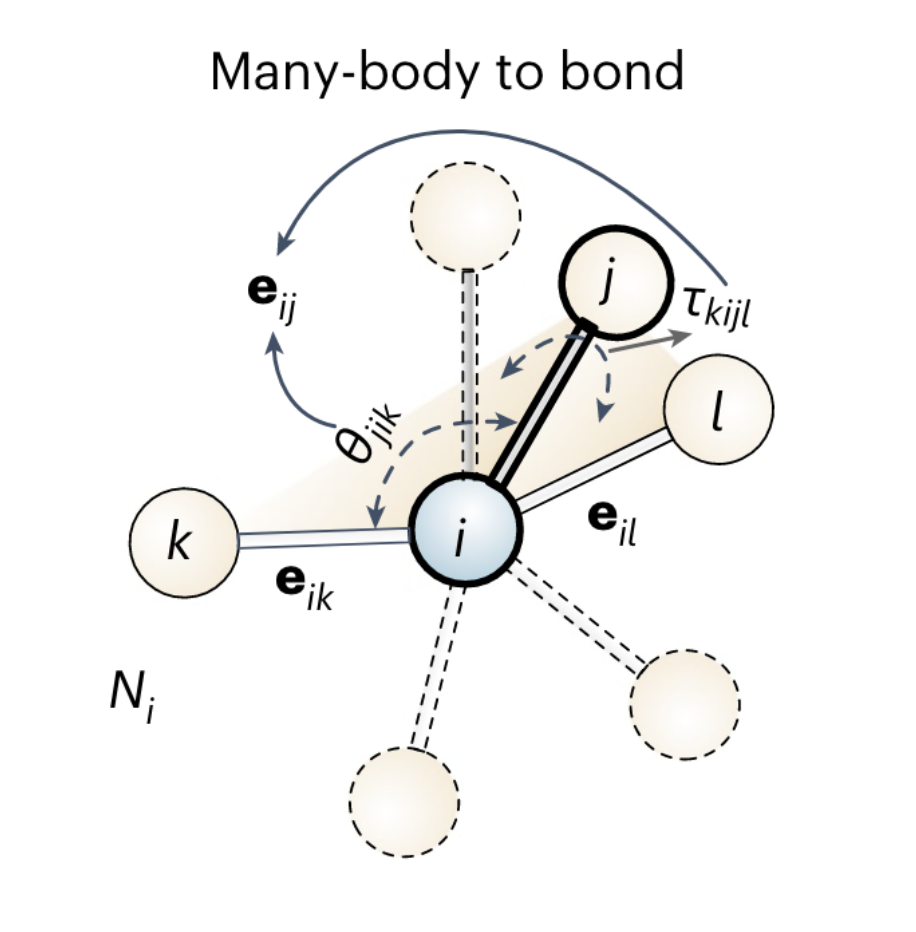
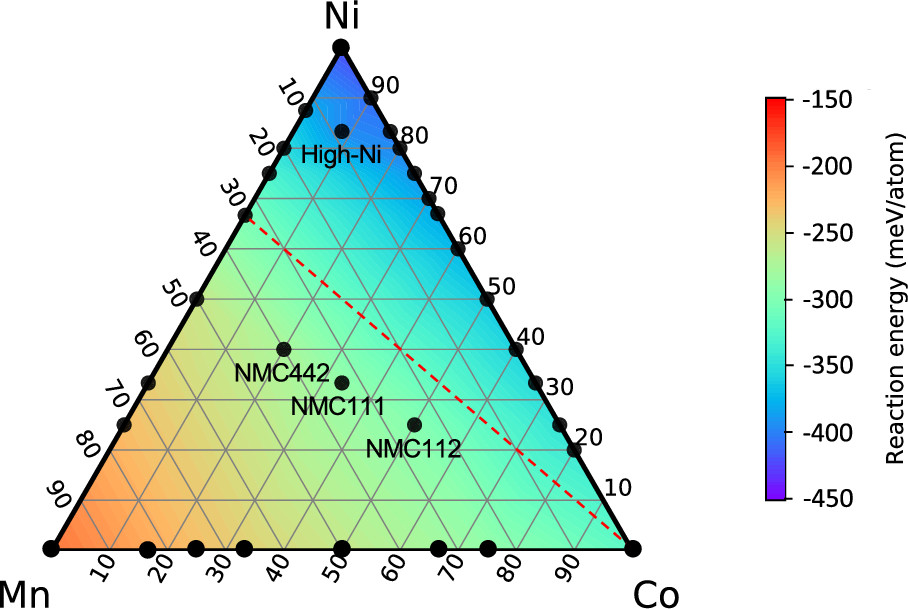
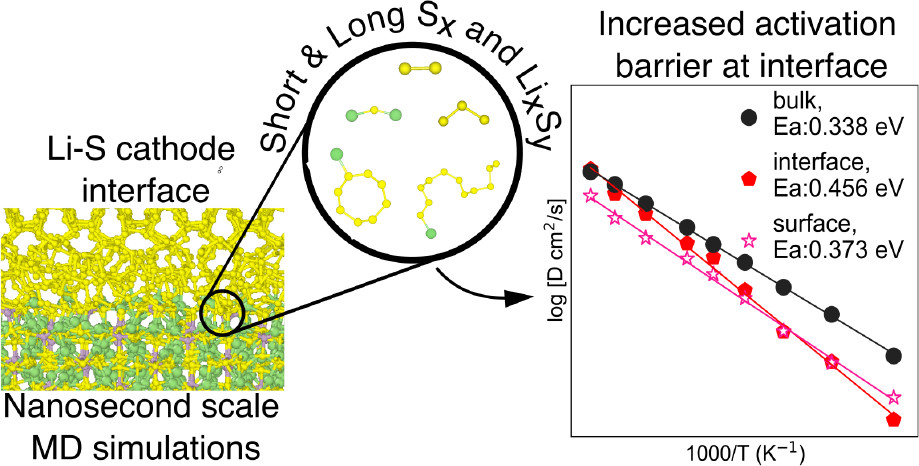
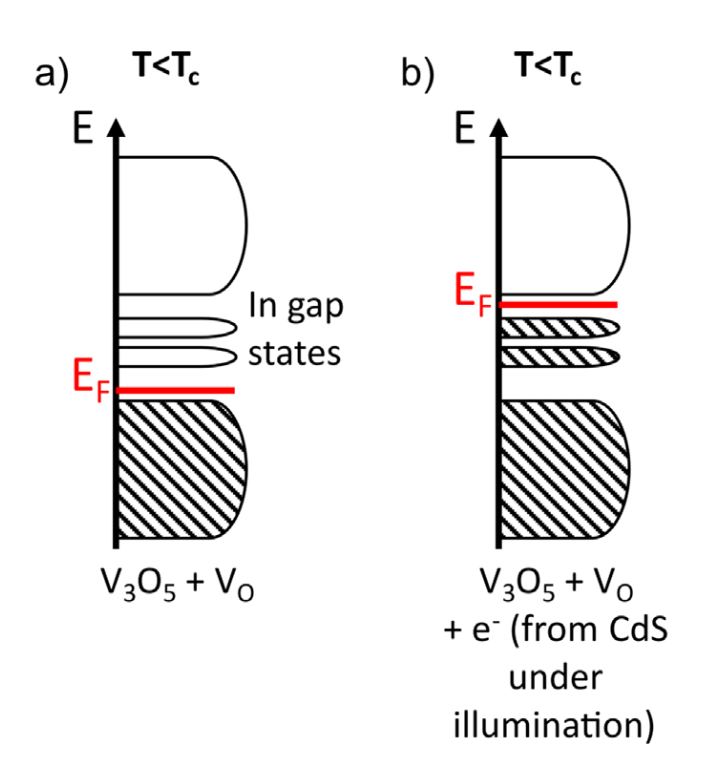
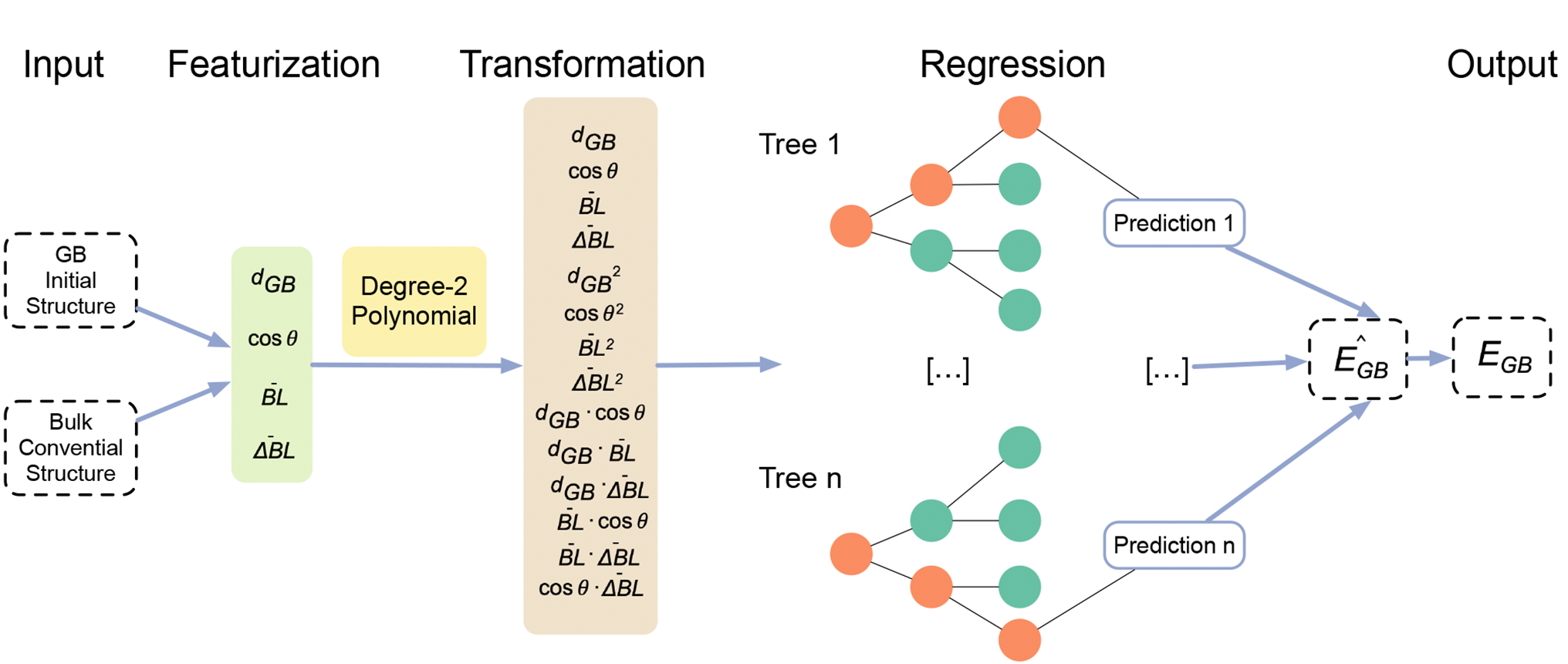
Multi-scale investigation of MoNbTi and TaNbTi MPEAs
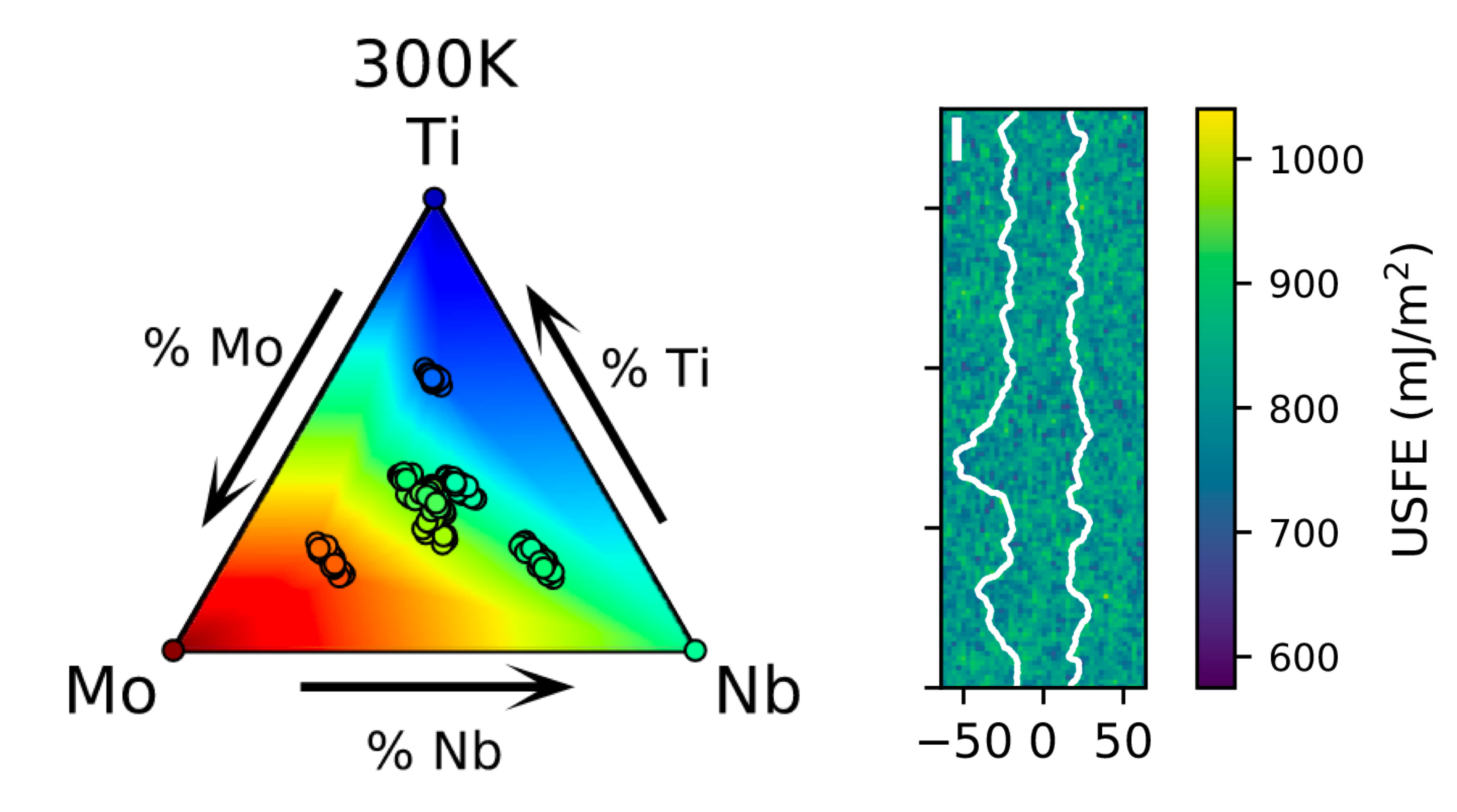
Hui’s swansong collaborative work on “Multi-scale investigation of short-range order and dislocation glide in MoNbTi and TaNbTi multi-principal element alloys” is now out in npj Computational Materials! This is a really exciting work that showcases an electron (DFT) to atom (machine learning interatomic potentials) to continuum (phase field dislocation dynamics) approach to the study of materials. It is a collaborative work between Hui and Lauren Fey of the group of Irene Beyerlein at UC Santa Barbara. Refractory multi-principal element alloys (RMPEAs) are promising materials for high-temperature structural applications. In this work, we performed an electron-to-atom-to-continuum study of the role of short-range ordering (SRO) on dislocation glide in the MoNbTi and TaNbTi RMPEAs. Monte carlo/molecular dynamics simulations with a moment tensor potential show that MoNbTi exhibits a much greater degree of SRO than TaNbTi and the local composition has a direct effect on the unstable stacking fault energies (USFEs). From mesoscale phase-field dislocation dynamics simulations, we find that increasing SRO leads to higher mean USFEs and stress required for dislocation glide. The gliding dislocations experience significant hardening due to pinning and depinning caused by random compositional fluctuations, with higher SRO decreasing the degree of USFE dispersion and hence, amount of hardening. […]