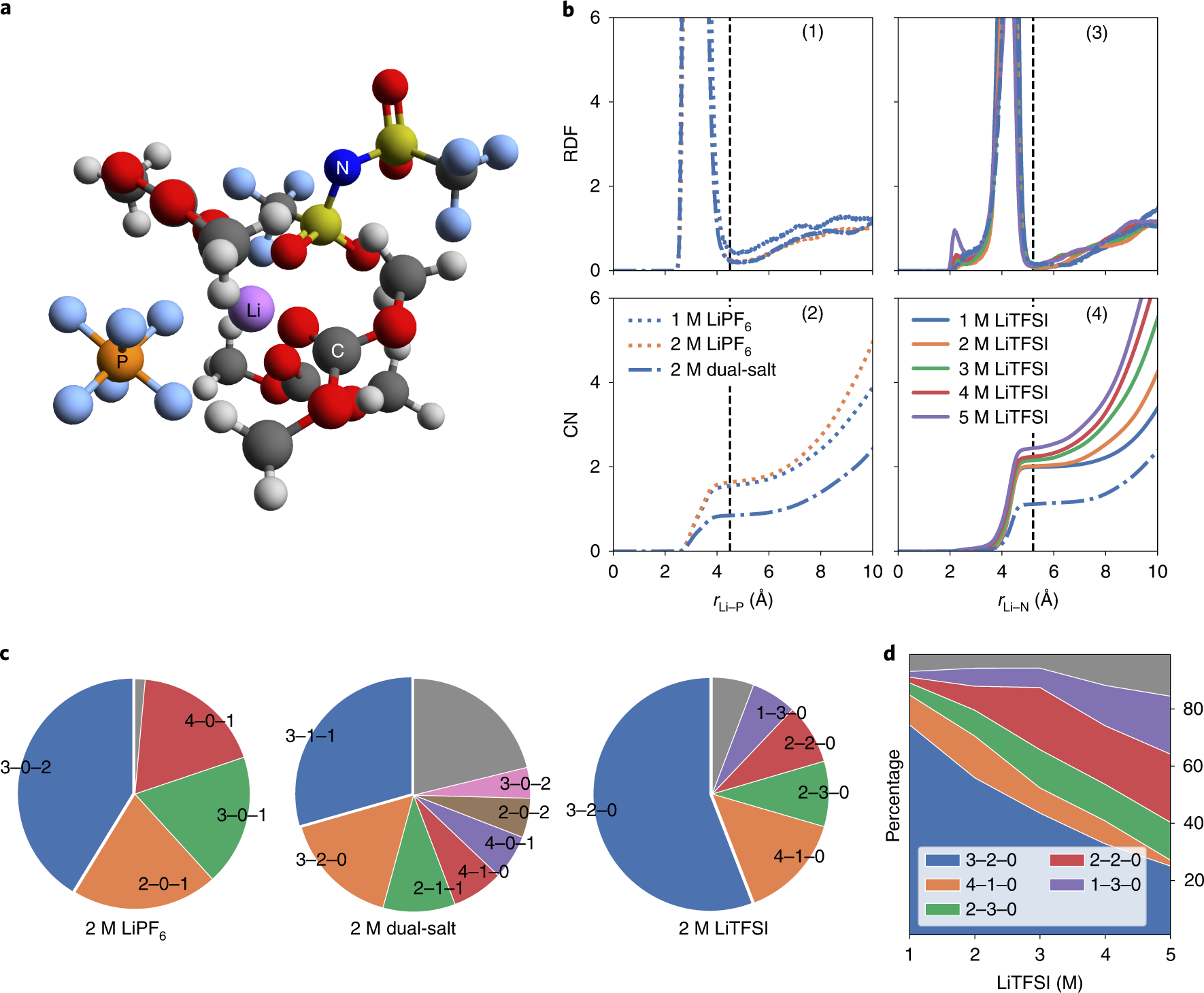
We are proud co-authors of an article in Nature Energy on “Ultrafast ion transport at a cathode–electrolyte interface and its strong dependence on salt solvation”. In this work, Bohua Wen from Prof Yet-Ming Chiang’s group at MIT performed electrodynamic measurements on single electrode particles to show that Li intercalation into NMC333 cathodes is primarily impeded by interfacial kinetics when using a conventional LiPF6 salt. Electrolytes containing LiTFSI salt, with or without LiPF6, exhibit about 100-fold higher exchange current density. Zhi Deng from the Materials Virtual Lab carried out MD simulations to show that TFSI preferentially solvates Li+ compared to PF6-, while still yielding a lower Li+ binding energy, explaining the observed ultrafast interfacial kinetics.
Check out this work at this link.