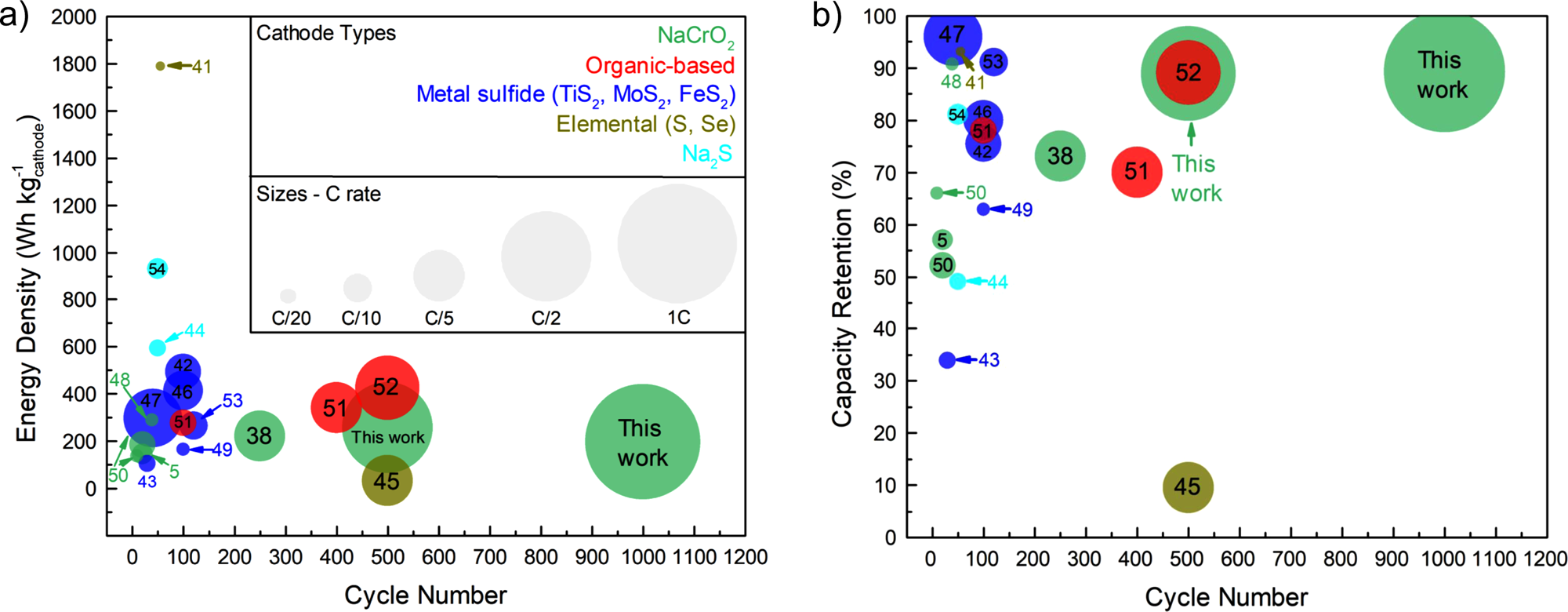
Our collaborative work with the Meng (UCSD) and Clement (UCSB) groups on the discovery of the Na3-xY1-xZrxCl6 (NYZC) ion conductor has just been published in Nature Communications. While rechargeable solid-state sodium-ion batteries (SSSBs) promise to bring about safer and more energy-dense energy storage, the poor interfacial stability between existing solid electrolytes and typical oxide cathodes has limited their long-term cycling performance and practicality. Using DFT calculations and MD simulations with a machine learning interatomic potential, Swastika Banerjee and Ji Qi from the Materials Virtual Lab identified NYZC as a promising new ion conductor that is both electrochemically stable up to 3.8 V vs. Na/Na+ and chemically compatible with oxide cathodes. NYZC’s ionic conductivity of 6.6 × 10−5 S/cm at ambient temperature, several orders of magnitude higher than oxide coatings, is due to abundant Na vacancies and cooperative MCl6 rotation. A SSSB comprising a NaCrO2 + NYZC composite cathode, Na3PS4 electrolyte, and Na-Sn anode exhibits an exceptional first-cycle Coulombic efficiency of 97.1% at room temperature and can cycle over 1000 cycles with 89.3% capacity retention at 40 °C. Check out our article at this link.



You must be logged in to post a comment.